

Flocculation and clarification are again employed to assist in solids separation. Precipitation, coprecipitation, and adsorption reactions generate suspended solids which must be separated from the wastewater. For example, hydroxide precipitation of ferric chloride can be used as the source of ferric oxide for coprecipitation and adsorption reactions. The removal of coprecipitive metals during precipitation of the soluble metals is aided by the presence of solid ferric oxide, which acts as an adsorbent during the precipitation reaction. Precipitation of metallic carbonate and sulfide species can be accomplished by the addition of calcium carbonate or sodium sulfide. For example, the precipitation of copper (Cu) hydroxide is accomplished by adjusting the pH of the water to above 8, using precipitant chemicals such as lime (Ca(OH) 2) or sodium hydroxide (N aOH). In general, these metals can be complexed to insoluble species by adding sulfide, hydroxide, and carbonate ions to a solution. Examples of such metals are cadmium, copper, lead, mercury, nickel, and zinc. Heavy metals are present in many industrial wastewaters. The rate and extent of this process is dependent upon the temperature and chemical characteristics of the wastewater, such as the concentration of metal initially present and other ionic species present, which can compete with or form soluble complexes with the target metal species. Particle growth involves the addition of more atoms or molecules into this particle structure. Nucleation is represented by the appearance of very small particle seeds which are generally composed of 10 –100 molecules.
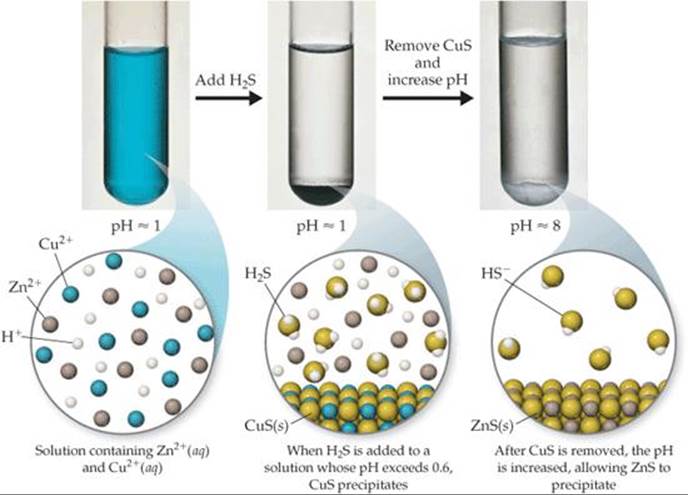
In theory, the precipitation process has two steps, nucleation followed by particle growth. Treatability studies must be performed to optimize the relevant variables, so that goals are met and costs minimized. Each of these variables directly influences treatment objectives and costs. They include the optimum pH, the type of chemical treatments used, and the number of treatment stages, as well as the temperature and volume of wastewater, and the chemical specifications of the pollutants to be removed. There are many different treatment variables that affect these processes. They are generally designed to precipitate trace metals to their solubility limits and obtain additional removal by coprecipitation and adsorption during the precipitation reaction. Precipitation processes are characterized by the solubility of the metal to be removed. Chemical precipitation includes two secondary removal mechanisms, coprecipitation and adsorption. The principle technology to remove metals pollutants from wastewater is by chemical precipitation.


 0 kommentar(er)
0 kommentar(er)
